A 69-year-old Chinese man was hospitalized for Common Terminology Criteria for Adverse Events (CTCAE) grade 2 acute kidney injury 8 months following treatment initiation with combination cytotoxic T-lymphocyte-associated protein 4 and programmed cell death protein-1 (PD-1) immune checkpoint blockade for treatment of adenocarcinoma of unknown primary. His medical history included immune checkpoint inhibitor (ICI)-induced adenohypophysitis and gastrointestinal reflux disease, for which proton pump inhibitor (PPI) omeprazole 20 mg twice daily was commenced 6 months prior. His serum creatinine (sCr) peaked at 299 ╬╝mol/L from a baseline of 100 ╬╝mol/L. Urinalysis was notable for pyuria (13 white blood cells/┬ĄL) without hematuria or significant proteinuria (urine protein-creatinine ratio, 0.27 g/g). C-reactive protein was elevated at 51.8 mg/L (reference range, 0.2ŌĆō9.1 mg/L). Serum complements were normal. Virology and autoimmune serologies such as hepatitis B, C, anti-double-stranded DNA, and antineutrophil cytoplasmic antibodies were negative. Ultrasonography revealed normal-sized kidneys without obstruction.
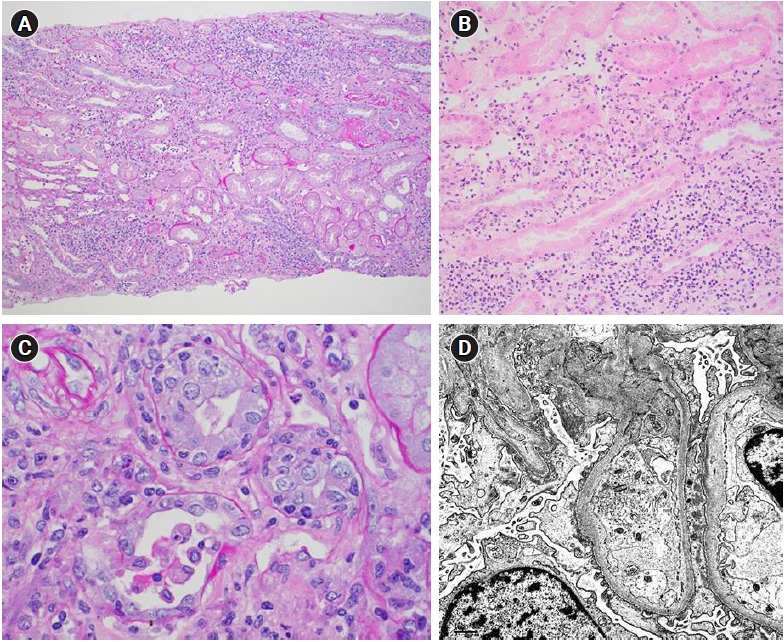
A kidney biopsy showed substantial interstitial inflammation and acute tubular injury consistent with ICI-induced acute tubulointerstitial nephritis (ICI-ATIN) (
Fig. 1A,
B). The diffuse tubulointerstitial mononuclear inflammatory infiltrates comprised lymphocytes, histiocytes, and some plasma cells, without tissue eosinophilia. No interstitial or intratubular granulomas were present. The glomeruli were relatively uninvolved. Interestingly, electron microscopy demonstrated moderate effacement of the foot processes suggestive of podocyte injury (
Fig. 1C).
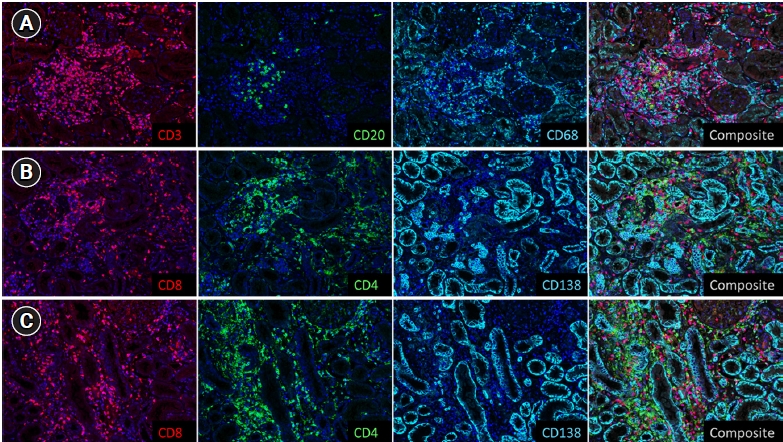
Immunohistochemistry stains showed a predominantly CD8+ lymphocytic infiltrate, macrophages, and scattered plasma cells (
Fig. 2). CD3+ T cells constituted about 80% of the total number of lymphocytes, while CD20+ B cells constituted the remaining 20%. About two-thirds of the T cells were CD8+, while one-third were CD4+. CD68+ histiocytes were fairly abundant. CD138+ plasma cells were the least abundant inflammatory cell type.
Oral prednisolone (PRL) was initiated at 60 mg (1 mg/kg) daily. Omeprazole was replaced with famotidine, a histamine type-2 receptor antagonist, due to the risk of ICI-ATIN potentiation with the continued use of a PPI. Mycophenolate mofetil (MMF) was added in view of inadequate treatment response and steroid-induced dysglycemia. A repeat biopsy at 6 weeks showed improving interstitial inflammation and marginal worsening of interstitial fibrosis. His sCr continued to improve, and PRL and MMF were gradually tapered. The single-agent PD-1 ICI was resumed 5 months after the initial ICI-ATIN episode due to rising tumor markers, with PRL maintained at 10mg daily. The sCr remained stable at 140 ╬╝mol/L 10 months after the ICI rechallenge.
Acute tubulointerstitial inflammation is the most common ICI-related kidney injury, either occurring alone or in combination with other glomerular pathologies [
1]. The histopathologic features of ICI-ATIN may resemble acute T cell-mediated renal allograft rejection. Interestingly, electron microscopy of our patientŌĆÖs biopsy revealed subclinical podocyte injury without manifestation of proteinuria. He reported no prior history of diabetes mellitus or use of antivascular endothelial growth factor therapies. The clinical significance of this finding is uncertain, but given reports of podocytopathies with ICI use, ongoing monitoring for the development of proteinuria is warranted.
Acknowledgments
We would like to acknowledge Dr. Joe Poh Sheng Yeong for his technical assistance with the images.
Figure┬Ā1.
Kidney biopsy showed acute tubulointerstitial nephritis.
(A, B) A diffuse tubulointerstitial mononuclear inflammatory infiltrate was noted, comprising lymphocytes, histiocytes, and some plasma cells, without tissue eosinophilia. No well-formed interstitial or intratubular granulomas are present (periodic acid-Schiff, ├Ś100). (C) Lymphocytic tubulitis (periodic acid-Schiff, ├Ś100). (D) Electron microscopy revealed a moderate degree of podocyte foot process effacement, affecting 30% to 40% of peripheral capillary wall surface area. This suggests an element of podocyte injury. No electron dense deposits were seen (├Ś6,000).
Figure┬Ā2.
Immunohistochemistry stains showed predominantly CD8+ lymphocytic infiltrate, macrophages, and scattered plasma cells (├Ś100).
(A) The staining results were analyzed using the Vectra imaging system. CD3+ T cells constituted about 80% of the total number of lymphocytes, while CD20+ B cells constituted the remaining 20%. B-lymphocytes formed small aggregates at times. About two-thirds of the T cells were CD8+, while one-third was CD4+. (B, C) Immunohistochemical stains were performed in two different cores of the kidney cortex for better representation. While CD8+ cells were diffusely and evenly distributed throughout the two cores, CD4+ cells tended to form small aggregates, probably in relation to the presence of B-lymphocytes. Scattered CD138+ plasma cells were observed. Overall, the cell types resembled those seen in acute T cell-mediated renal allograft rejection.
Reference
1. Hong MH. Nephrotoxicity of cancer therapeutic drugs: focusing on novel agents.
Kidney Res Clin Pract 2021;40:344ŌĆō354.













 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















